Learning Outcomes
By the end of this lesson, students should be able to:
i. Explain the concept of nucleophilic acyl substitution reactions involving carboxylic acid derivatives.
ii. Identify the reagents and conditions for nucleophilic acyl substitution reactions of different derivatives.
iii. Describe the mechanisms of nucleophilic acyl substitution reactions for acyl halides, acid anhydrides, esters, and amides.
iv. Provide examples of nucleophilic acyl substitution reactions involving various nucleophiles and carboxylic acid derivatives.
Introduction
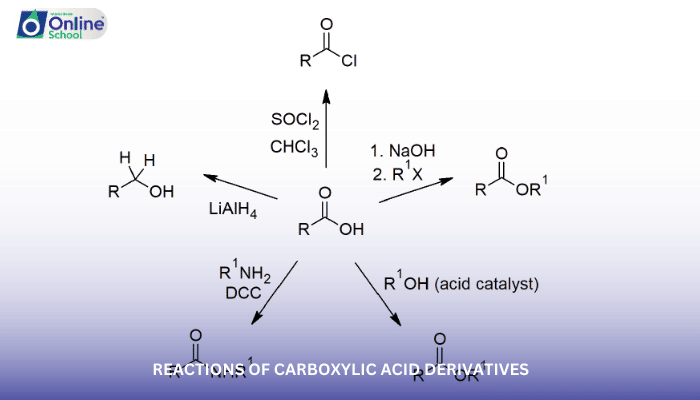
Carboxylic acid derivatives, with their reactive carbonyl groups, readily undergo nucleophilic acyl substitution reactions. These reactions involve the attack of a nucleophile, an electron-rich species, on the carbonyl carbon, leading to the formation of a new bond and the displacement of the leaving group attached to the carbonyl carbon.
i. Nucleophilic Acyl Substitution Reactions with Acyl Halides
Acyl halides, the most reactive carboxylic acid derivatives, are highly susceptible to nucleophilic acyl substitution reactions. The halide atom (-Cl, -Br, or -I) acts as the leaving group, and a wide range of nucleophiles, including alcohols, amines, and cyanide ions, can participate in these reactions.
ii. Nucleophilic Acyl Substitution Reactions with Acid Anhydrides
Acid anhydrides, possessing two carbonyl groups, can also undergo nucleophilic acyl substitution reactions. The nucleophile attacks one carbonyl carbon, and the leaving group is the carboxylate anion (-COO-) from the other carbonyl group.
iii. Nucleophilic Acyl Substitution Reactions with Esters
Esters, generally less reactive than acyl halides and acid anhydrides, can still undergo nucleophilic acyl substitution reactions under certain conditions. Strong nucleophiles, such as lithium aluminum hydride (LAH) or sodium borohydride (NaBH4), can attack the carbonyl carbon, leading to the formation of alcohols.
iv. Nucleophilic Acyl Substitution Reactions with Amides
Amides, the most stable carboxylic acid derivatives, are generally resistant to nucleophilic acyl substitution due to their strong resonance stabilization. However, under specific conditions, such as acidic or alkaline media, amides can undergo hydrolysis or reactions with highly reactive nucleophiles.
Nucleophilic acyl substitution reactions play a pivotal role in the chemistry of carboxylic acid derivatives, providing a versatile pathway for the synthesis of various compounds with diverse applications. Understanding the reactivity of these derivatives and the factors influencing their reactions is essential for designing synthetic routes and predicting the outcomes of nucleophilic acyl substitution reactions.